006. Intuitive Reaction Simulation and Process Optimisation using Compunetics
By Connor Taylor on September 13, 2020
One major benefit to conducting kinetic analysis is that when the kinetic parameters of a reaction are known, the reaction can be simulated at different starting concentrations and temperatures. This allows the chemist to predict with confidence how the reaction should proceed upon changing these variables, rather than running further laboratory experiments which would cost significantly more time and resources to conduct. When reaction simulation is used in conjunction with Compunetics Investigator, the chemical process of interest can be effortlessly optimised in silico to determine optimum operating conditions, without the need to even enter the laboratory.
In a particular chemical process, the yield of the pharmaceutical building block, C, is to be optimised (maximised). 2,4-dichloropyrimidine (starting material) A, reacts with morpholine, B, to form either the 4-substituted product, C, or the 2-substituted product, D. These products can then further react with another equivalent of morpholine to produce the bis-substituted product, E. Each of these reactions form hydrochloric acid as a side product that is neutralised by an excess of base:
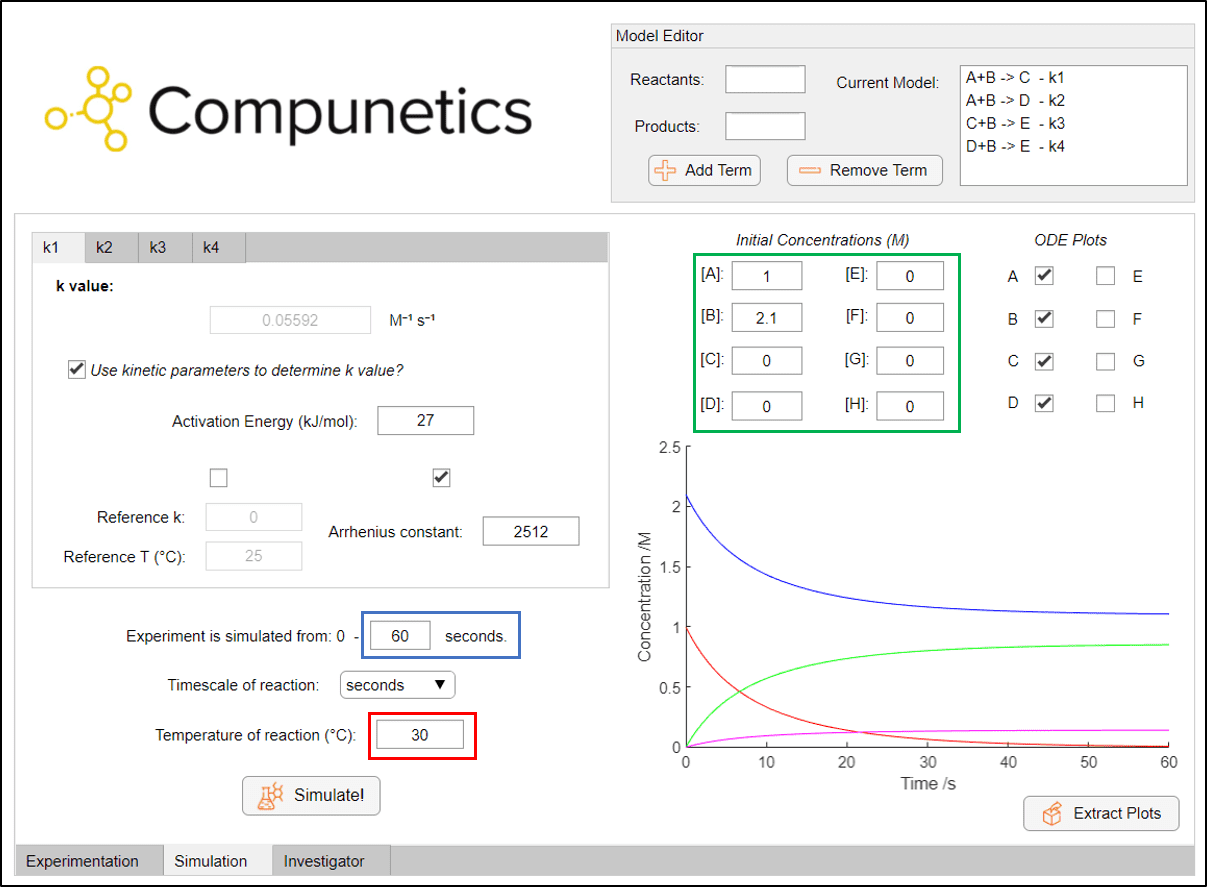
The corresponding kinetic information can be quickly and easily entered into Compunetics. Reactions can then be simulated dynamically by changing the experimental simulation time (blue box), the temperature of the reaction (red box) and the initial concentrations (green box):
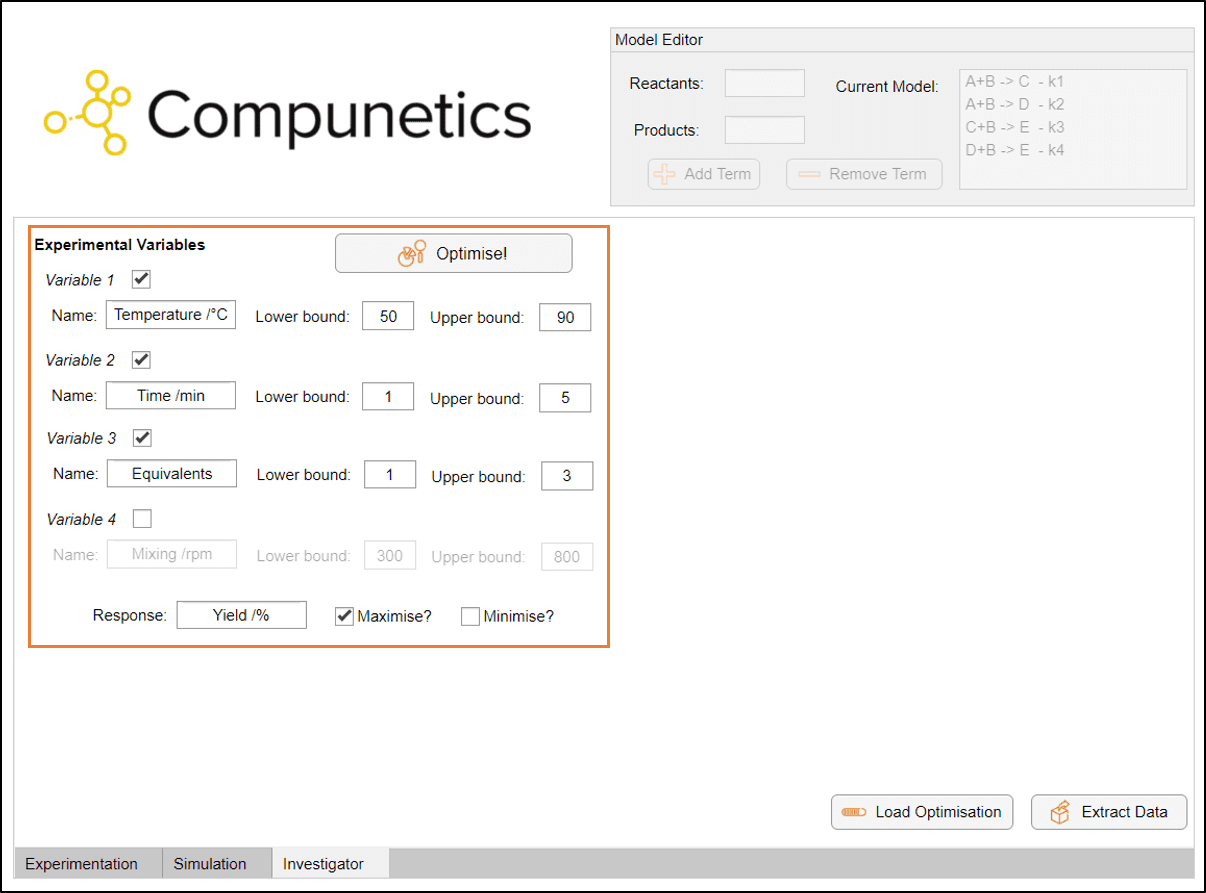
This tool can of course be used for exploratory purposes, but we want to optimise our process. Therefore, using the Investigator tool, we can utilise a machine learning algorithm to optimise our chemical process via these simulations. In this instance, we are optimising for a maximum yield, with a particular set of experimental bounds (orange box). For this optimisation, we would like to know the optimum operating conditions when exploring: Temperature (50 - 90 °C), Reaction Time (1 - 5 minutes) and Equivalents of B (1 - 3):
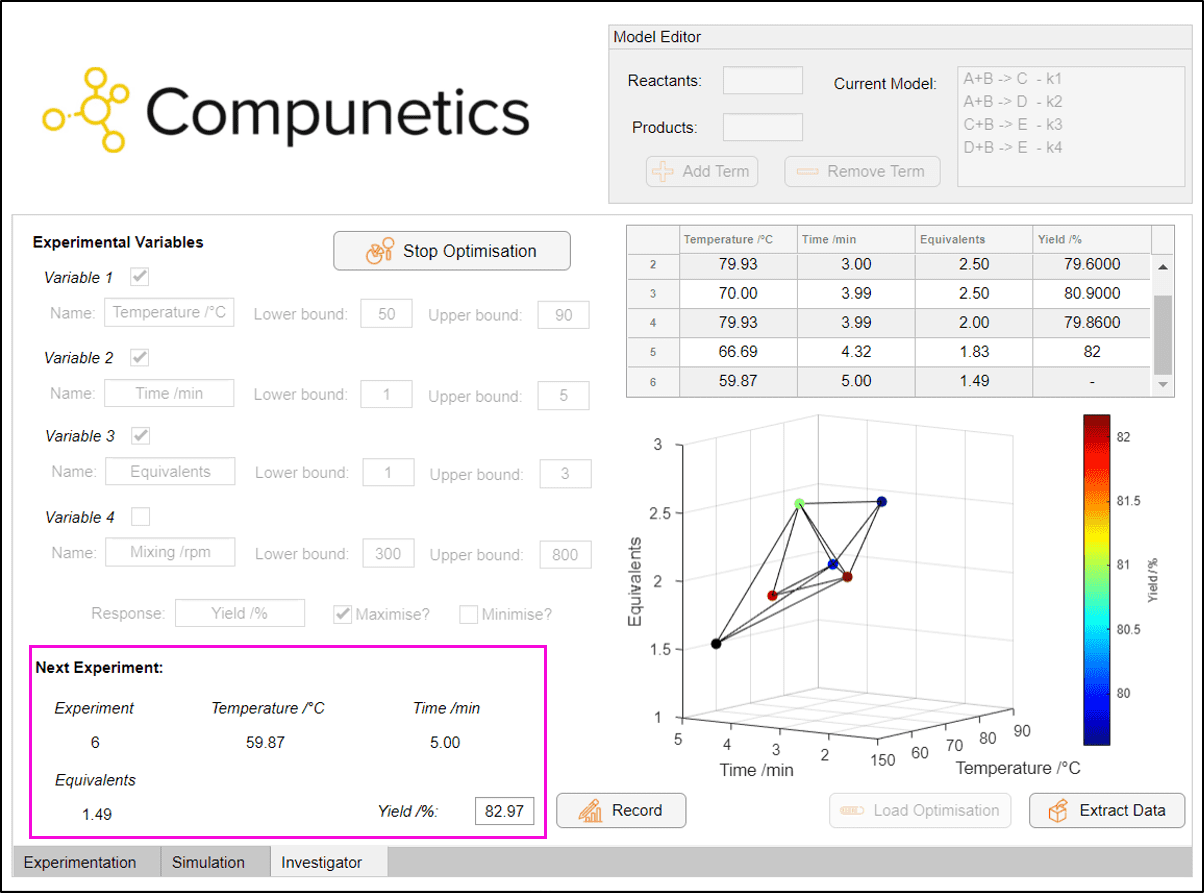
Upon clicking 'Optimise!', we are requested to complete the required experiments for the optimisation to proceed (purple box). This algorithm was built to replace the worst-performing vertex of each geometric prism (Simplex) to iterate towards the optimum - which is both highly efficient and highly effective at optimising problems:
The Investigator terminated by optimising the process in silico in 9 experiments, identifying an optimum yield of 84% at 50 °C, 4 minutes reaction time and 1 equivalent of B. This tool can be used in a variety of real and simulated experimental cases to quickly identify optimum reaction conditions, especially in cases where it's preferential to conduct fewer experiments. Notably, this technique has been shown to consistently out-perform one-factor-at-a-time (OFAT) optimisations.
It has been shown that reaction simulation and process optimisation are both effortless when using Compunetics. It has long been the case that these techniques were reserved for the modelling experts, the coders and the physical-organic chemists. This is no longer the case, as Compunetics can be used by anyone to easily simulate reactions. It is now more important than ever that chemists can utilise experimental simulation as part of their synthetic toolkit, as we enter the post-COVID laboratory of 2020 - where simulations can be run from home to replace real experiments, to supplement thesis chapters and papers in a refreshing way.
1: Reizman, B. J. and Jensen, K. F., An Automated Continuous-Flow Platform for the Estimation of Multistep Reaction Kinetics. Organic Process Research & Development, 2012, 16, 11, 1770 - 1782.